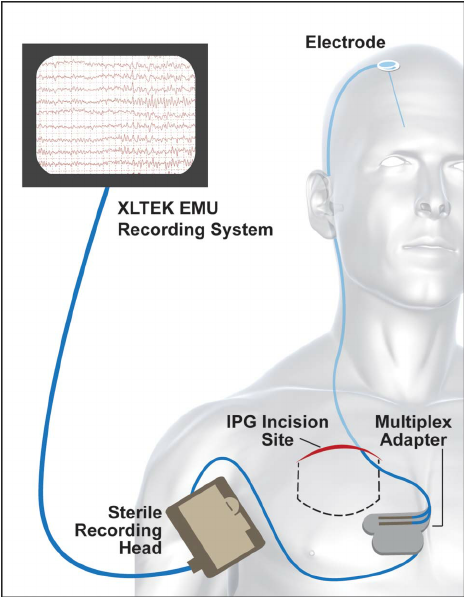
Through UMN organizations such as the UMN Medical School and Academic Health Center, we have the resources needed to execute a human feasibility test for your new product while our Internal Review Board ensures that research is conducted ethically and safely.
Medical School
The University's Medical School has researchers and specialists with expertise in neuroscience, magnetic resonance imaging, and disciplines across the health sciences to help you get the first human data for your therapy.
Talk to Greg Peterson and Dwight Nelson to get connected to a department or a particular physician.
Academic Health Center
Part of the AHC, the Office of Discovery and Translation (ODAT) connects innovators with UMN experts to help translate research ideas on the path to human health. Their expertise includes:
- Pre-clinical study design
- Pre-clinical toxicology
- Regulatory strategy and guidance
- Biostatistics
- Product development
- Chemical manufacturing and controls (CMC)
- Clinical trial design and conduct
Reach out to Dwight Nelson to connect to researchers through ODAT.
Internal Review Board:
The University's biomedical Internal Review Board (IRB) meets every other week to ensure ethical conduct of human research.
- Of the 82% of applications which receive expedited or non-committee review due to exemption or minimal risk, the median project moves forward in 6-10 weeks.
- For the fraction of projects reviewed via full committee, the team receives a response within a median time of 2-3 months.
Reach out to Greg Peterson for perspective on the IRB.
Further Resources:
Check our Resources page to learn about more resources that may be helpful at the first-in-human phase.